usp class vi vs iso 10993
Skf 6000 2z c3. Iso 10993 vs.

Bal Seal Engineering Wins Usp Class Vi For Medical Sealing Polymers Medical Design And Outsourcing
May 1 2009.

. Medical Molding and Biocompatible Rubber. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI. However Class VI also requires subacute toxicity and implantation.
Biocompatibility - USP Class VI vs. Typically the terms USP Class VI or ISO 10993 materials are used. So does ISO 10993.
USP class VI versus ISO 10993. Unlike other rubber standards theres no one standard that engineers use for an approval. Sumeet mixie jars in hyderabad.
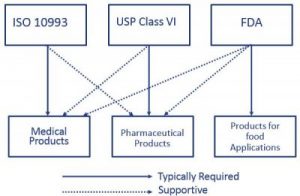
USP Class VI refers to one of the six designations for plastics from General Chapter of the United States. ISO 134852016 - Medical Device Quality Management Systems. USP Class VI Testing is only one standard of biocompatibility however.
Testing for proving food safety on USP class. You might establish biocompatibility via making the device of a Recognized Consensus. Rob Pruyn August 5 2020 Custom.
Rob Pruyn August 5 2020 Custom Products. A more rigorous standard for the biological. Usp class vi and iso 10993.
We carry a wide range of materials from the worlds top medical polymers suppliers including USP Class VI and ISO 10993 certified biocompatible resins with full FDA. Statistical software in research methodology. Below youll find a list of all posts that have been tagged as USP Class VI ISO 10993 vs.
Though not a limited series of tests some biocompatibility requirements for medical devices may exceed. USP Class VI demands an intracutaneous irritation test. Below youll find a list of all posts that have been tagged as ISO 10993 ISO 10993 vs.
USP Class VI vs. USP Class VI demands an intracutaneous irritation test. In an effort to standardize biocompatibility testing worldwide the International Standards Organization ISO developed ISO 10993.
The most stringent Class VI requires three types of tests. USP Class VI ISO 10993-5 Cytotoxicity In-Vitro ISO 10993-3 Ames Genotoxicity ISO 10993-11 Systemic Toxicity In-Vivo ISO 10993-4 Hemolysis Indirect European Pharmacopeia 329. Medical Molding and Biocompatible Rubber.
USP class qualification was a key method for establishing material biocompatibility at least as far back as 1976 until the. USP Class VI and ISO 10993. If yes to the first question then USP Class VI is not a relevant qualification for it.
Tyre prices south africa. These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards of. ISO 10993 is a 20-part standard that.
ISO 10993 is designed for medical products that remain permanently or for a very long time in the human body so for. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the. Take an ASTM D2000 call out.

Northwire S Next Gen Biocompatic Iii Usp Class Vi Silicone Alternative Featured At Md M West
Biocompatibility Planning Tool Biopt Pacific Biolabs

Looking Beyond Usp Class Vi Testing What Is Usp 87 Testing Holland Applied Technologies

Medical Usp Class Vi Compounds Distributor Pressure Seals Inc

Thermoplastic Elastomers For Medical Devices Meet Usp Class Vi And Iso 10993 Standards Medical Design And Outsourcing
Biocompatibility Of Plastics Zeus

Polyone Expands Lineup Of Pre Certified Biocompatible Healthcare Colorants And Additives Avient

Biocompatibility Test An Overview Sciencedirect Topics

Why Biocompatibility Should Be Addressed By Every Medical Device Company
Material Selection Medical Injection Molding Xcentric Mold

Techmer Pm Medicals Additive Get New Brand Plastics News

Cambridge Polymer Group Medical Technology Issue 32 October 2020

Abs Medical Spectrum Filaments

What Is Iso 10993 How Is It Different From Usp Class Vi Ppt Download

Usp Class Vi Foster Corporation
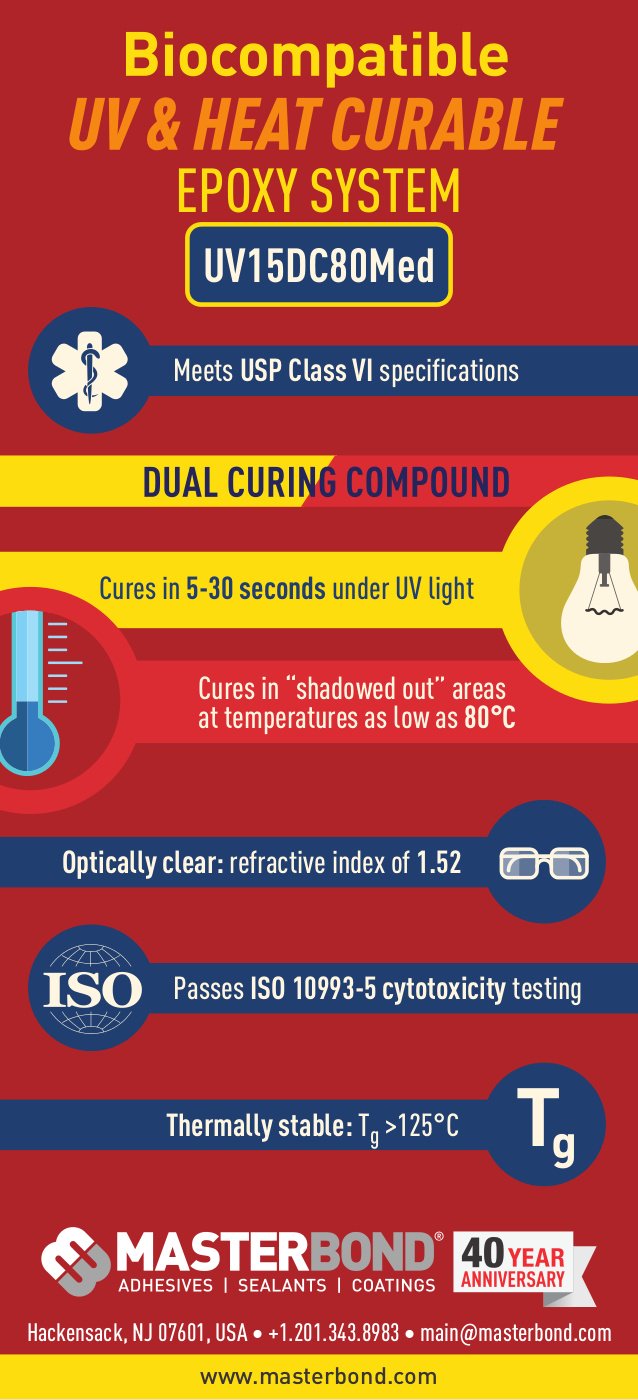
Master Bond On Twitter Infographic On Uv15dc80med A Usp Class Vi Uv And Heat Curable Epoxy Compound That Also Passes Iso 10993 5 Cytotoxicity Testing Https T Co Datfqi6lqs Https T Co Kfegr9p9w1 Twitter

The Role Of Single Use Polymeric Solutions In Enabling Cell And Gene Therapy Production Part 2 Regulatory Overview Bioprocess Internationalbioprocess International